Osteoarthritis is a degenerative joint disease that affects the lives of more than 32 million Americans. Post-traumatic osteoarthritis (PTOA), a type of osteoarthritis caused by injuries, comprises 10% of diagnoses and results in more than $3 billion in healthcare costs each year. In more than half of these cases, patients have suffered anterior cruciate ligament (ACL) damage, leading to PTOA within 10–15 years. However, PTOA has no effective therapeutic protocols to slow or stop its progression except for over-the-counter analgesics.
Trauma-induced cartilage injury is difficult to treat because cartilage has limited capacity to regenerate; cartilage is an avascular, aneural, and alymphatic tissue that has a complex structure with unique mechanical demands. Currently, upon suffering a cartilage or joint injury, patients rest until healed and potentially undergo surgery if there exists a repairable tear in the adjacent tissue to prevent further joint destabilization. However, the efficacy of these repair surgeries in preventing the onset of PTOA is minimal. After a few months, an estimated 40–60% of those patients suffer the onset of PTOA.
The disease is marked by swelling, bone spurs, instability, and most of all, pain. These symptoms progressively worsen with no option to arrest them. Eventually, a patient is required to undergo joint replacement surgery. Neither a disease-modifying osteoarthritis drug nor a non-surgical cure presently exist for these patients. During the disease progression, non-steroidal anti-inflammatory drugs (NSAIDs) and steroids can attenuate PTOA-related pain, but they have no effect on disease progression, and their use can be limited greatly by their potentially severe side effects. Thus, a disease-modifying treatment that addresses the root cause is desperately needed.
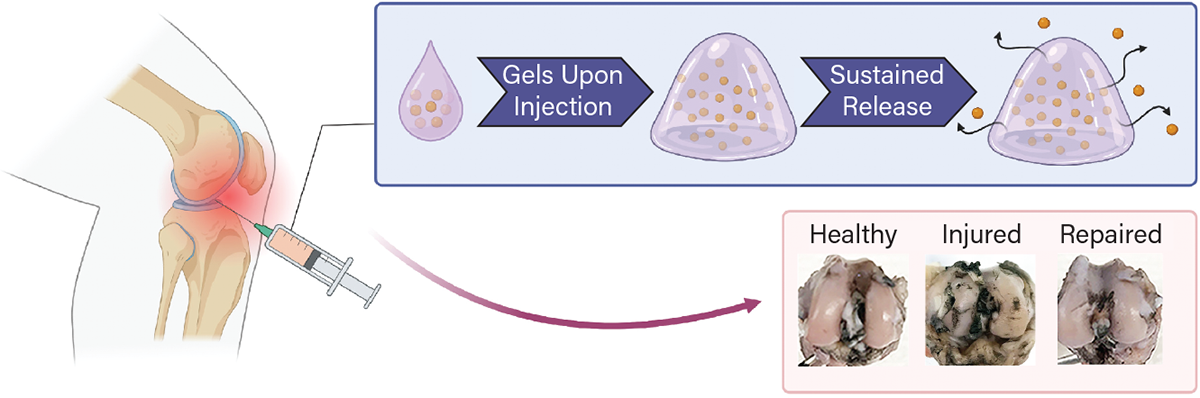
▲ A research team from New York Univ. has developed a minimally invasive injectable hydrogel therapy for treating post-traumatic osteoarthritis (PTOA). The E5C-Atsttrin hydrogel undergoes a sol-gel phase transition upon intraarticular injection. Photos from eight weeks post-injection demonstrate cartilage repair.
With the support of the U.S. National Science Foundation (NSF) and Dept. of Defense, Professor of Chemical and Biomolecular Engineering Jin Kim Montclare and her group at New York Univ. (NYU) have developed a minimally invasive injectable hydrogel therapy for treating PTOA.
Injectable hydrogels undergo a temperature-dependent phase transition from solution (sol) to gel. If this transition is tuned so that gelation occurs under physiological conditions, then the gel can remain localized in the area of injection and serve as a reservoir for releasing a therapeutic. While conventional hydrogels have been composed of synthetic polymers, Montclare’s novel work focused on the development of protein-engineered “phase transitioning” gels. The team developed E5C, a recombinant protein block polymer consisting of elastin-like polypeptides (E) and the coiled-coil domain of cartilage oligomeric matrix protein (C) — both of which can be found in cartilage tissue. The resulting E5C encapsulates and delivers Atsttrin, a protein-engineered derivative of the growth factor progranulin. Atsttrin is essentially a truncated progranulin that is more potent in treating inflammatory arthritis. NYU has patent coverage for both the E5C and the Atsttrin.
This combination of two engineered proteins results in E5C-Atsttrin, which is a solution that, upon injection into the joint, becomes a hydrogel at physiological conditions. This single injection is able to deliver the therapeutic over a prolonged time and treats cartilage for regeneration while also protecting bone quality.
Using a rabbit PTOA model, it was shown that the E5C-Atsttrin gel protects against PTOA onset and biodegrades within eight weeks post-injection in the joint. The hydrogel provides an optimized biomechanical and biochemical environment leading to the suppression of inflammation and support of cartilage regeneration.
“This novel biomaterial represents the first and only solution to modifying the progression of osteoarthritis, effectively stopping the pathogenesis, and represents a new minimally invasive drug delivery system for degenerative joint diseases,” says Montclare. Future work on investigating the preclinical dosing and toxicology will be explored toward commercialization of this work.
This research was funded in part by NSF’s Division of Materials Research.
This article was prepared by the National Science Foundation in partnership with CEP.

Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
