The field of heterogeneous catalysis is undergoing transformational change. In most traditional, supported transition metal catalysts, the active reaction sites are located on nanoscale clusters or particles. Breaking the nanoscale barrier and driving active site dispersion down to the single atom is a major research frontier for catalysis. This frontier is becoming a reality via single-atom catalysts (SACs), which have atomically dispersed metals that are supported on high-surface-area materials. SACs offer the advantages of well-defined sites and high selectivity combined with the high stability and easy separation typically found with solid catalysts.
SACs have the potential to provide unique catalytic performance due to intimate electronic interactions between the isolated active site and the ligand supports. Recent studies have shown promise for diverse industrial applications such as acetylene hydrochlorination, ethylene hydroformylation, and the electrochemical oxygen reduction reaction. A general limitation for SACs has been the lack of scalable synthesis methods that can produce high loadings of isolated active sites with the stability required for industrial implementation. At the same time, the ability to use well-established “off-the-shelf” industrial catalyst supports is of critical importance for adoption of such materials.
At the Center for Rational Catalyst Synthesis (CeRCaS), an Industry-University Cooperative Research Center (IUCRC) funded by the U.S. National Science Foundation (NSF), a new approach has been developed to produce high loadings of isolated atoms without the need for supports with tailored anchoring sites.
The new method is called “Chelate Fixation,” or CheFi, and derives from the insight that, in current catalyst preparation methods, water on the support surface and hydrated catalyst precursors form into nanodroplets during drying. These nanodroplets cause the metal precursors to aggregate, even prior to activation, into nanoparticles. In CheFi, a high-boiling chelating agent is dissolved into an aprotic solvent and, upon addition to water, binds to the metal and the support. The solvent modifies the surface tension of the drying mixture, while the chelating agent isolates the metal precursors during activation with hydrogen. The chelating agent can then be removed by either high-temperature desorption/decomposition or a washing step.
The difference between the typical and CheFi approaches for making SACs is clear in the representative scanning transmission electron microscopy (STEM) images for 3.5 wt% Pt/VXC-72 (~0.5 Pt/nm2) catalyst treated in hydrogen at 300°C. The sample prepared by strong electrostatic adsorption (SEA) yields 1–2 nm particles, while CheFi showed significantly higher dispersion with isolated atoms and only ultrasmall clusters. Indeed, metal loadings of at least 1 atom/nm2 can be prepared in this way. For a high-surface-area support, such as NORIT ROX activated carbon, 30 wt% Pt loading can be achieved, with high densities of isolated atoms even after reductive treatments at elevated temperatures. For example, STEM images after treatment in hydrogen at 170°C show primarily isolated atoms, along with some ultrasmall Pt clustering. A sample with similar Pt loading prepared using typical industrial preparation methods will primarily consist of supported nanoparticles.
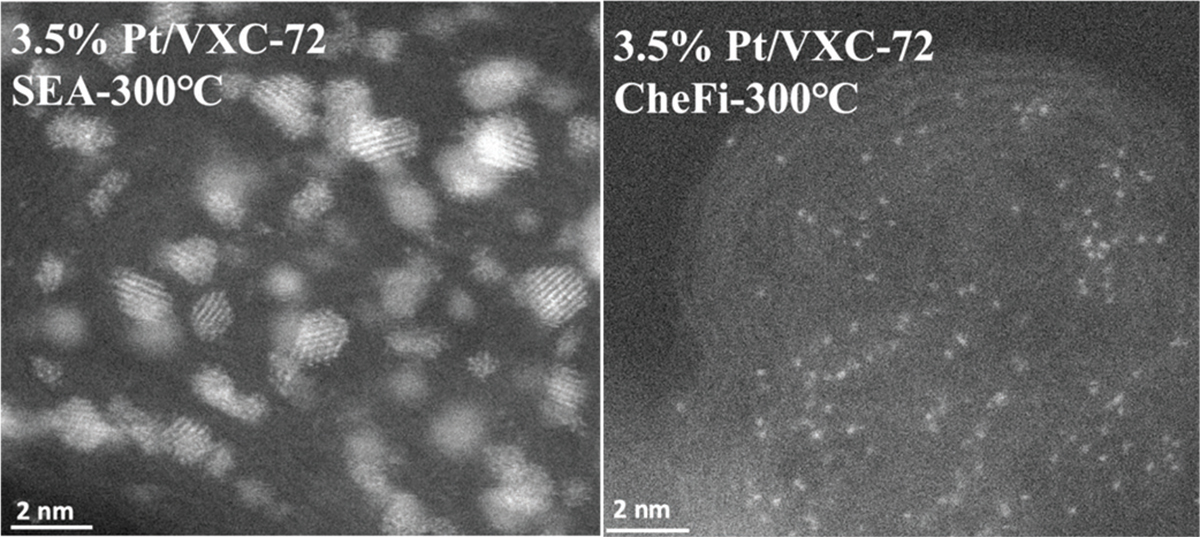
▲ A heterogeneous catalyst consisting of 3.5 wt% Pt on Vulcan (VXC-72) carbon-black was prepared by strong electrostatic adsorption (SEA) (left) and CheFi (right). CheFi was able to achieve a much greater Pt dispersion, including a high density of SACs.
The CheFi approach has been applied to numerous carbon (activated, black, diamond) and oxide (silica, titania, alumina, magnesia) supports, and with Pt, Pd, Ir, and Ru metals. Batch sizes of 20 g have been prepared, and with the same basic setup, batches of one kilogram or more are possible. Just as exciting is the potential for this approach to enable new catalyst technology based on well-accepted industrial supports. ExxonMobil’s Stuart Soled states, “The area of supported SACs has been gaining a huge interest in the catalysis community over the last number of years. What has been desperately needed is a simple preparation method that can be utilized by researchers in the area, and which would lend itself to practical scale-up. It is toward that end that the CheFi methodology has shown some early promise. ExxonMobil and other member companies in this IUCRC are really excited to see where this can lead.”
This technology was funded through the NSF Industry-University Cooperative Research Centers (IUCRC) program.
This article was prepared by the National Science Foundation in partnership with CEP.

Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
